Duchenne muscular dystrophy (DMD) is a progressive genetic disorder that affects the functioning of the skeletal and cardiac muscles and could even be fatal. Clinical applications of cell therapies for DMD face numerous challenges, limiting the potential therapeutic benefits. A group of Japanese researchers has now shown that injecting mesenchymal stromal cells from amnion into mice can halt the progression of DMD. This new therapeutic strategy may greatly benefit patients with DMD.
Duchenne muscular dystrophy (DMD) is a common progressive muscular disorder. It is an X-chromosome-linked disorder that gradually weakens various muscle groups including those supporting the heart, thus resulting into early death. While steroids are widely used to regulate severe inflammation in patients with DMD, they are known to cause serious adverse effects. In theory, the injection of mesenchymal stromal cells—supporting immune-regulation—can be used to slow down the progression of muscular dystrophy. However, its therapeutic effects require further investigation.
A recent study examined the effect of injecting human-amnion-derived mesenchymal stromal cells into DMD model mice. The results are quite promising and show long-term improvement of skeletal and cardiac muscle function in treated mice. This paper was published in Volume 14 Issue 108 of the journal Stem Cell Research & Therapy on April 27, 2023. The research team included Prof. Takashi Okada and Dr. Yuko Nitahara-Kasahara from The Institute of Medical Science, The University of Tokyo; and Masahiro Hayashi and Tomoyuki Nakaishi from Kaneka Corporation.
“We focused on amnion-derived mesenchymal stromal cells (AMSCs), which have unique characteristics such as non-invasive isolation, mitotic stability, ethical acceptability, and a low risk of immune reaction” remarks Prof. Takashi Okada, from The Institute of Medical Science, The University of Tokyo.
AMSCs are known to exert immunomodulatory effects. Put simply, when intravenously injected into the target organism, they are able to suppress certain immune responses and also exhibit anti-inflammatory properties, thus making them ideal candidates for various therapeutic applications. Moreover, AMSCs are abundant in amnion, and they can be harvested using non-invasive techniques.
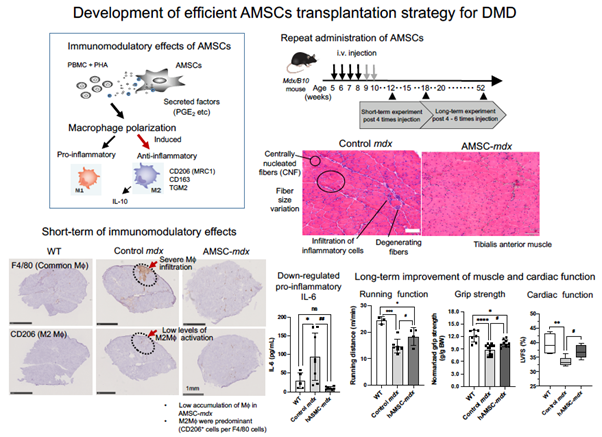
First, the research team isolated human AMSCs (hAMSCs) from amnion that were aseptically obtained from consenting pregnant women undergoing cesarean delivery. Next, they intravenously injected these hAMSCs into a DMD mouse model (mdx mouse) and studied their immunomodulatory effects. They also analyzed the expression of anti-inflammatory M2 macrophage markers on peripheral blood mononuclear cells (PBMCs) co-cultured with hAMSCs. When systemically injected into the affected mice, hAMSCs secrete various factors influencing the polarization of macrophages, or specialized white blood cells, from the pro-inflammatory “M1” state to the anti-inflammatory “M2” state.
The team monitored the hAMSC-treated and untreated mdx mice using a variety of tests. These included standard blood tests, histological examinations, spontaneous wheel-running activities, assessment of grip strength, and echocardiography. Treatment with hAMSCs showed significant promise, especially when delivered during the early phase of DMD. For instance, the proportion of anti-inflammatory M2 macrophages increased significantly in hAMSC-treated mdx mice. Quite notably, mdx mice that received the hAMSC treatment showed a better grip strength than the untreated mdx mice.
The treated mdx mice showed a transient downregulation of serum creatine kinase after repeated systemic hAMSC injections. "The downregulation of serum creatine kinase is usually associated with the suppression of muscle damage,” comments Dr. Nitahara‑Kasahara. The hAMSC treatment also resulted in the regulation of muscle damage following inflammation. In addition, it improved the histological appearance of the previously impacted skeletal muscles. Quite notably, the hAMSC-treated mdx mice also covered a greater distance per minute than their untreated counterparts during standard activity tests. Most importantly, the cardiac muscles of the treated mdx mice exhibited better functioning than those of the untreated mdx mice.
“This treatment strategy could provide therapeutic benefits to patients with DMD. This study is presumably the first to report the therapeutic benefits of AMSCs for severe muscular disease,” muses Prof. Okada. Although the published work still needs clinical translation, it nevertheless paves the way for a highly potent therapeutic intervention targeting DMD. Meanwhile, let’s thank the researchers for their strong and the early stages of stellar biomedical contributions!
Amnion-derived mesenchymal stromal cells (AMSCs) may benefit patients with Duchenne muscular dystrophy (DMD)
AMSCs polarize macrophages, exert anti-inflammatory effects, facilitate muscle recovery, and improve cardiac function in a mouse model of DMD, according to a new study by researchers from Japan. They have also demonstrated the development of an efficient transplantation strategy for AMSCs for DMD.
Reference
Journal:
Stem Cell Research & Therapy
Title of original paper:
Immunomodulatory amnion‑derived mesenchymal stromal cells preserve muscle function in a mouse model of Duchenne muscular dystrophy
Stem Cell Research & Therapy
Title of original paper:
Immunomodulatory amnion‑derived mesenchymal stromal cells preserve muscle function in a mouse model of Duchenne muscular dystrophy
DOI:
10.1186/s13287-023-03337-0
Authors:
Yuko Nitahara Kasahara1, Soya Nakayama2, Koichi Kimura3, Sho Yamaguchi2, Yuko Kakiuchi1, Chikako Nito4, Masahiro Hayashi2, Tomoyuki Nakaishi2, Yasuyoshi Ueda2 and Takashi Okada1
Affiliations:
1Division of Molecular and Medical Genetics, Center for Gene and Cell Therapy, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-Ku, Tokyo, 108-8639, Japan
2Regenerative Medicine and Cell Therapy Laboratories, Kaneka Corporation, Kobe, Japan
3Department of Laboratory Medicine, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
4Laboratory for Clinical Research, Collaborative Research Center, Nippon Medical School, Tokyo, Japan
About Dr. Takashi Okada
Dr. Takashi Okada works as a Professor at The Institute of Medical Science, The University of Tokyo. Prof. Okada’s research group primarily works in the area of viral-vector-based gene and cell therapy. Prof. Okada has secured numerous research grants as a principal investigator and co-investigator for research projects focusing on therapeutic interventions for neuromuscular diseases, cancer, exon skipping, optogenetic control of macaque temporal neurons, and more. He has also published his work in various peer-reviewed scientific journals.UTokyo PEOPLE
Dr. OKADA Takashi
Media contact
Affiliation: Project Coordination Office, The Institute of Medical Science, The University of Tokyohttps://www.ims.u-tokyo.ac.jp/imsut/en/index.html