Cell senescence is a state of permanent cell cycle arrest that was initially defined for cells grown in cell culture. It plays a key role in age-associated organ dysfunction and age-related diseases such as cancer, but the in vivo pathogenesis is largely unclear.
A research team led by Professor Makoto Nakanishi of the Institute of Medical Science, the University of Tokyo, generated a p16-Cre ERT2 -tdTomato mouse model (*1) to characterize in vivo p16 high cells (*2) at the single-cell level.
They found tdTomato-positive p16 high cells detectable in all organs, which were enriched with age. They also found that these cells failed to proliferate and had half-lives ranging from 2.6 to 4.2 months, depending on the tissue examined.
Single-cell transcriptomics in the liver and kidneys revealed that p16 high cells were present in various cell types, though most dominant in hepatic endothelium and in renal proximal and distal tubule epithelia, and that these cells exhibited heterogeneous senescence-associated phenotypes.
Further, elimination of p16 high cells ameliorated nonalcoholic steatohepatitis-related hepatic lipidosis and immune cell infiltration.
These results were published in " Cell Metabolism " on September 18, 2020.
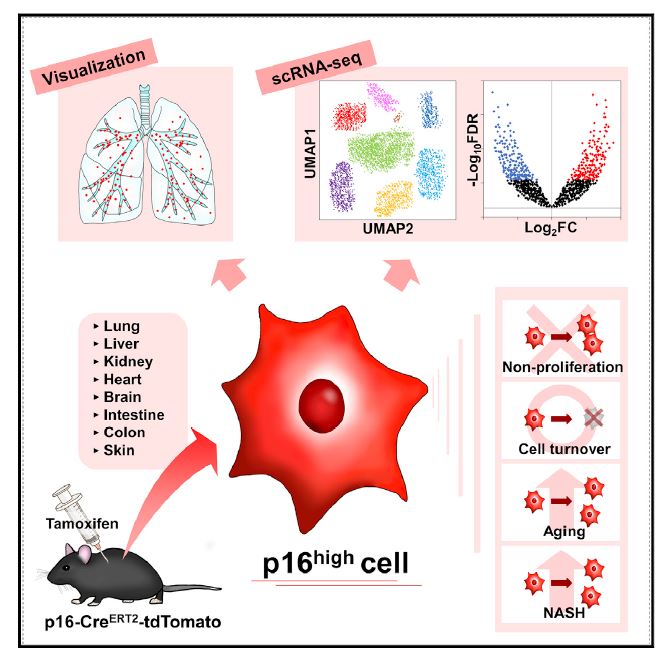
Figure : The research team generate a p16-Cre ERT2 - tdTomato mouse model to uncover the in vivo dynamics and properties of p16high cells. Single-cell RNA-seq analyses of various tissues from early middle-aged p16-CreERT2-tdTomato mice reveal that p16high cells exhibit heterogenous senescence-associated phenotypes, while elimination of p16high cells ameliorates steatosis and inflammation in a NASH model.
There were a variety of senescent cells in the kidney, lung, liver, heart, brain・・・・
According to the research team, tamoxifen (TAM,*3) was administered to middle-aged mice to investigate the location of senescent cells. What they found was that they could detect these cells in all organs they investigated such as kidney, lung, liver, heart, brain…etc.
In addition, they investigated how senescent cell presence changed with age, and found that individual senescent cells did not proliferate, but the number of senescent cells in all organs increased significantly with aging.
It was also shown that non-alcoholic steatohepatitis (NASH,*4) was significantly improved when senescent cells were removed from the liver and kidneys. This is an interesting result from the perspective of NASH prevention and treatment.
For details of the research, please see the paper.
In addition, they investigated how senescent cell presence changed with age, and found that individual senescent cells did not proliferate, but the number of senescent cells in all organs increased significantly with aging.
It was also shown that non-alcoholic steatohepatitis (NASH,*4) was significantly improved when senescent cells were removed from the liver and kidneys. This is an interesting result from the perspective of NASH prevention and treatment.
For details of the research, please see the paper.
Contribution to the further elucidation of the causes of human aging and the development of anti-aging therapies
These results have shown that senescent cells in vivo are diverse depending on the type of progenitor cell and the stimulus.
And their new mouse model and single-cell analysis provide a powerful resource to enable the discovery of previously unidentified senescence functions in vivo.
Lead Scientist Professor Nakanishi said “ These are the first results in the world showing the comprehensive transcriptome profiles of individual senescent cells in vivo, and we hope that it will contribute to the further elucidation of the causes of human aging and the development of anti-aging therapies”.
And their new mouse model and single-cell analysis provide a powerful resource to enable the discovery of previously unidentified senescence functions in vivo.
Lead Scientist Professor Nakanishi said “ These are the first results in the world showing the comprehensive transcriptome profiles of individual senescent cells in vivo, and we hope that it will contribute to the further elucidation of the causes of human aging and the development of anti-aging therapies”.
Funding
This study was supported by MEXT/JSPS KAKENHI under grant numbers JP26250027, JP22118003, and JP16K15239; AMED under grant numbers JP17cm0106122, JP17fk0310111, and JP17gm5010001; Ono Medical Research Foundation, Princess Takamatsu Cancer Research Fund, and RELAY FOR LIFE JAPAN CANCER SOCIETY to M.N.; JP18H05026m, JP19H03431, JP20H04940, and JP16H06276 (AdAMS) to YJ; 17H06095 to MY and HF and 19H03413 to EAS; JST CREST A3A28043 to MY; the World Premier International Research Center Initiative (WPI) from MEXT to MY; and AMED JP20am0401411 to HRUSO was supported by JSPS as a Research Fellow.
Research Notes
(*1) p16-Cre ERT2 -tdTomato mouse model
Mice inserting CreERT2 into the endogenous Ink4a locus with Rosa26-CAG-lsl-tdTomato. In this mouse model, p16 high cells are labelled with tdTomato (red fluorescence) in vivo by tamoxifen administration.
(*2) p16 high cells
Cells expressing p16Ink4a at high level. Most of p16 high cells are thought to be senescent cells in vivo.
(*3) Tamoxifen (TAM)
Tamoxifen and its metabolite 4-hydroxytamoxifen are selective estrogen response modifier, acting as an estrogen antagonist. They can be used for activation of CreERT2 (tamoxifen-inducible Cre-ERT2 fusion protein).
(*4) Non-alcoholic steatohepatitis (NASH)
Advanced form of non-alcoholic fatty liver disease caused by buildup of fat in the liver. NASH is liver inflammation and damage by a buildup of fat.
Mice inserting CreERT2 into the endogenous Ink4a locus with Rosa26-CAG-lsl-tdTomato. In this mouse model, p16 high cells are labelled with tdTomato (red fluorescence) in vivo by tamoxifen administration.
(*2) p16 high cells
Cells expressing p16Ink4a at high level. Most of p16 high cells are thought to be senescent cells in vivo.
(*3) Tamoxifen (TAM)
Tamoxifen and its metabolite 4-hydroxytamoxifen are selective estrogen response modifier, acting as an estrogen antagonist. They can be used for activation of CreERT2 (tamoxifen-inducible Cre-ERT2 fusion protein).
(*4) Non-alcoholic steatohepatitis (NASH)
Advanced form of non-alcoholic fatty liver disease caused by buildup of fat in the liver. NASH is liver inflammation and damage by a buildup of fat.
About the research
1) Journal Article
Satotaka Omori#, Teh-Wei Wang#, Yoshikazu Johmura*, Tomomi Kana, Yasuhiro Nakano, Taketomo Kido, Etsuo A Susaki, Takuya Nakajima, Shigeyuki Shichino, Satoshi Ueha, Manabu Ozawa, Kisho Yokote, Soichiro Kumamoto, Atsuya Nishiyama, Takeharu Sakamoto, Kiyoshi Yamaguch, Seira Hatakeyama, Eigo Shimizu, Kotoe Katayama, Yasuhiro Yamada, Satoshi Yamazak, Kanako Iwasaki, Chika Miyoshi, Hiromasa Funato, Masashi Yanagisawa, Hiroo Ueno, Seiya Imoto, Yoichi Furukawa, Nobuaki Yoshida, Kouji Matsushima, Hiroki R Ueda, Atsushi Miyajima, and Makoto Nakanishi* ”.Generation of a p16 reporter mouse and its use to characterize and target p16hight cells in vivo” Cell Metabolism.
(#:co-first author, *:co-corresponding author)
DOI:10.1016/j.cmet.2020.09.0006
URL : https://doi.org/10.1016/j.cmet.2020.09.0006
(#:co-first author, *:co-corresponding author)
DOI:10.1016/j.cmet.2020.09.0006
URL : https://doi.org/10.1016/j.cmet.2020.09.0006
2) Publication Journal
Cell Metabolism
https://www.cell.com/cell-metabolism/home
https://www.cell.com/cell-metabolism/home